April 22, 2020
ALPCO’s Calprotectin Validation Assistance Program
Whether your clinical laboratory is transitioning over to the ALPCO Calprotectin Chemiluminescence ELISA from another calprotectin assay or starting to run the test in-house for the first time, validation is the most crucial step in the assay implementation process. We understand that validating a new ELISA can be a constant balancing act of keeping current laboratory operations functioning while also planning and performing the required testing. As a result, ALPCO has developed a Calprotectin Validation Assistance Program to alleviate the stress of implementing our test in your clinical lab. Our program provides your organization with the necessary training, materials, and guidance to guarantee successful implementation of the Calprotectin Chemiluminescence ELISA with minimal impact on current operations.


 Download a PDF of our Calprotectin Validation Assistance Program overview to share with your lab team members.
Download Program Overview
Download a PDF of our Calprotectin Validation Assistance Program overview to share with your lab team members.
Download Program Overview
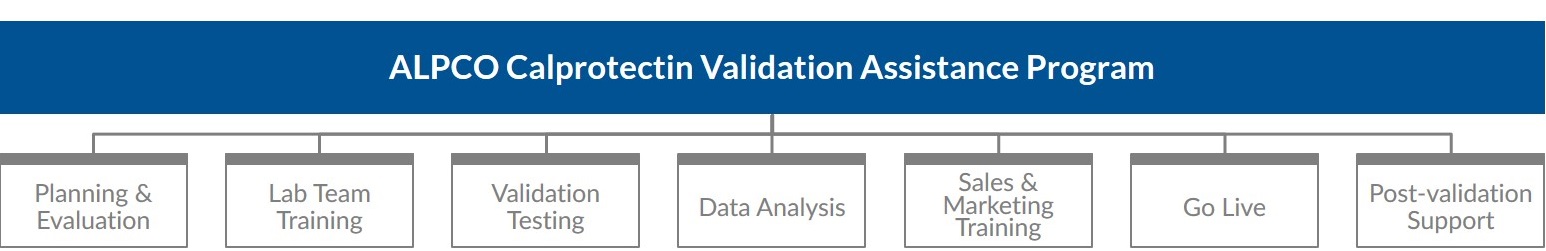
Calprotectin Validation Assistance Program Features
Our goal is to assist your clinical lab in successfully implementing our calprotectin test so you can help your gastroenterology clients more accurately diagnose patients with IBD or IBS. ALPCO’s program provides your lab with:- A knowledgeable Field Applications Scientist for remote and on-site assistance with validation testing
- Customized training for lab, sales, and marketing teams
- Necessary testing materials including ELISA kits and stool extraction devices
- Access to a sample panel
- A STELLUX® 4400 Chemiluminescence Plate Reader
- Guidance to generate test and method change notifications
- Continual post-validation technical support, refresher training, and new hire training
How Our Validation Program Benefits Your Lab
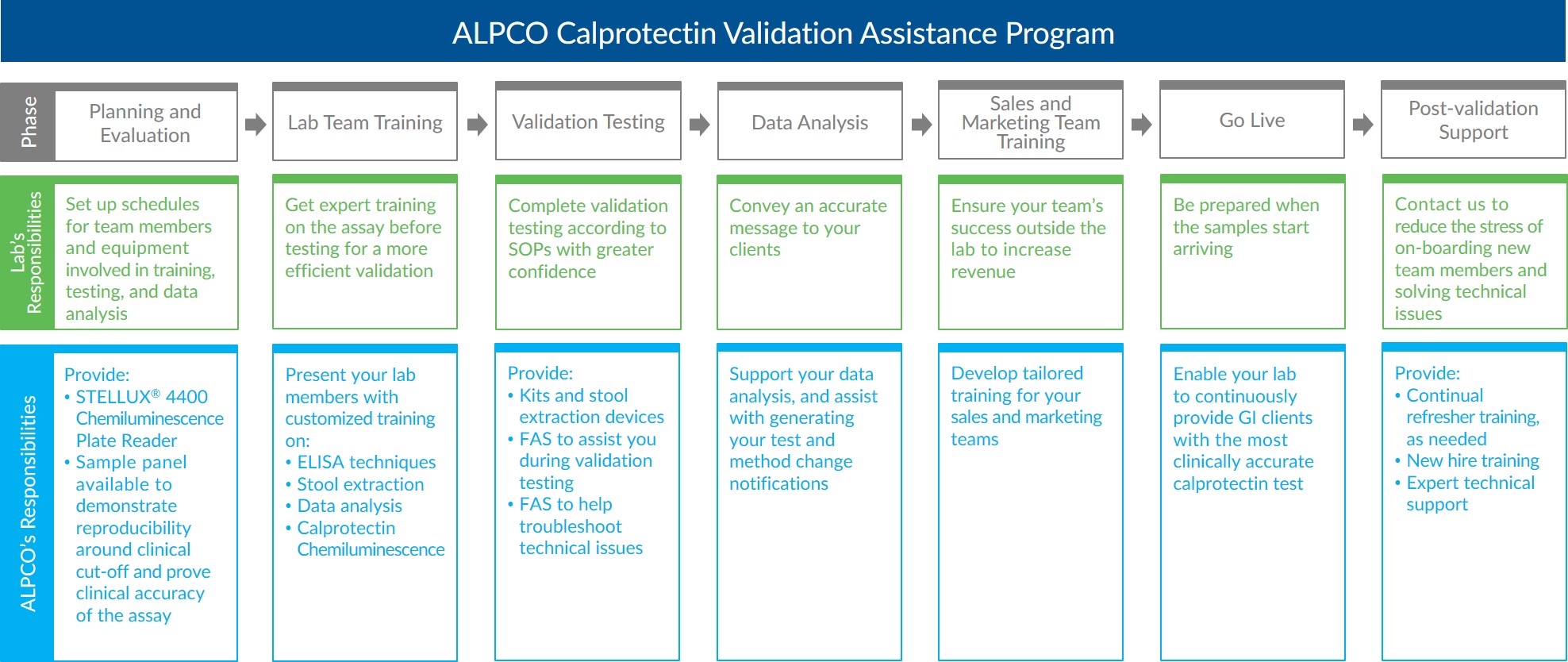
Our program is designed to support your clinical lab through every stage of validation and implementation of the Calprotectin Chemiluminescence ELISA to overcome resource and budget constraints during planning, validation, go live, and beyond.
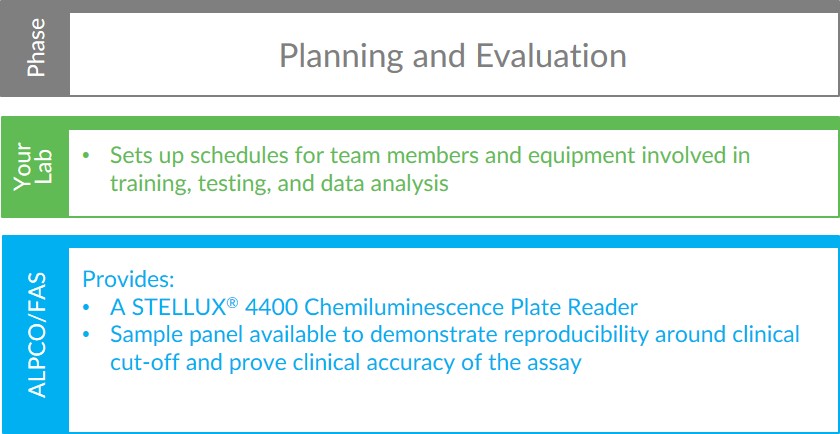
Planning and Evaluation
The Calprotectin Validation Assistance Program begins at the planning and evaluation phase. During this phase, your lab organizes schedules for the team members and equipment needed for training, testing, and data analysis. ALPCO assists your lab by providing a STELLUX® 4400 Chemiluminescence Plate Reader for the initial assay evaluation. By utilizing our plate reader, your lab can evaluate the Calprotectin Chemiluminescence ELISA without borrowing equipment from existing workflows and continue current operations without interruption. Additionally, your lab will have access to an ALPCO sample panel for the initial assay evaluation. The panel is designed to demonstrate inter-laboratory reproducibility and clinical accuracy, as well as reproducibility around the clinical cut-off. ALPCO’s Field Application Scientist (FAS) and product management team will coordinate the delivery and set up of a STELLUX® 4400 Chemiluminescence Plate Reader and the sample panel to your lab. If needed, arrangements can also be made for ALPCO’s FAS to be onsite during the initial evaluation to provide support and guidance to your team.
Customized Lab Team Training
ALPCO offers your lab customized training in order to equip each member of the team with the skills needed to successfully run our calprotectin test and any other ALPCO assays of interest. Our Field Applications Scientist will work with your lab to understand the team’s needs. By training your lab team before assay validation, the testing phase will be more efficient and streamlined. Areas of training led by our highly experienced FAS may include:- ELISA techniques on manual and automated systems
- Stool sample extraction methods
- Data analysis
- Running the Calprotectin Chemiluminescence ELISA

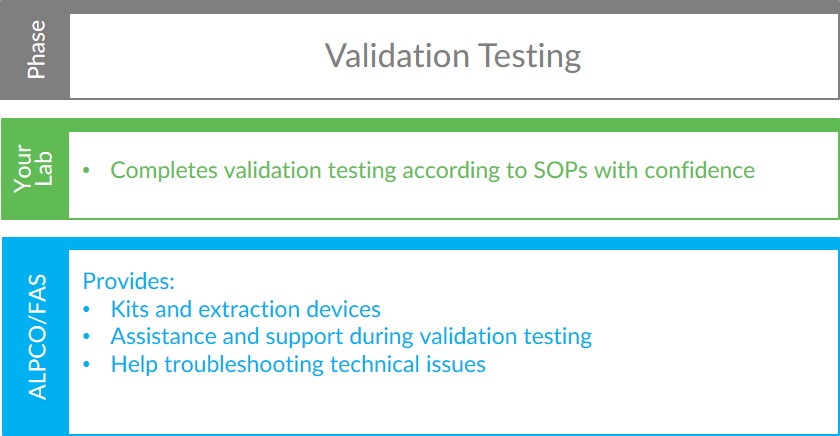
Validation Testing
During the assay validation testing phase, your team will complete testing according to the internal standard operating procedures dictated by your lab’s certification requirements. ALPCO will provide the Calprotectin Chemiluminescence ELISA kits and stool extraction devices needed to complete the planned testing. While your team is validating the assay, our FAS will be available to support your lab with expert technical assistance. With the training and materials provided by ALPCO, your lab team will be able to confidently validate our FDA cleared calprotectin test.
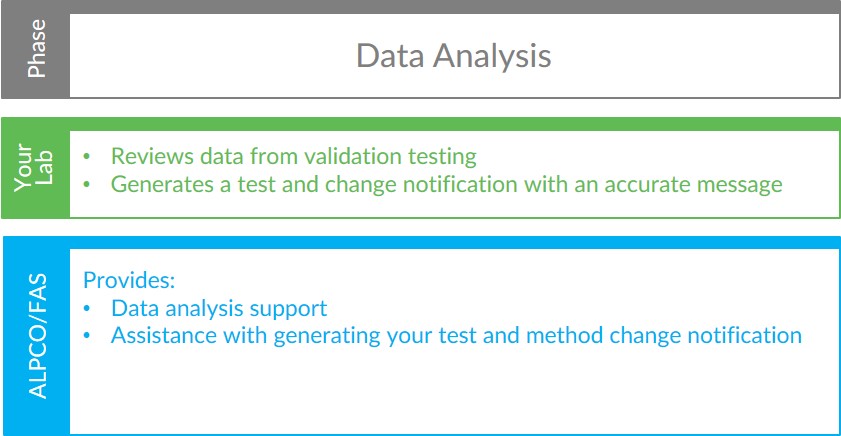
Data Analysis
After assay validation testing is complete, your lab will need to analyze the data produced to complete the process. Our FAS will be available during this phase to guide your team through template creation in your software of choice. Additionally, our FAS will assist with generating the test and method change notification relative to switching to ALPCO’s calprotectin test. With our support, your team will be able to convey an accurate message to your GI clients, ensuring they are on board with transitioning to the Calprotectin Chemiluminescence ELISA and that they understand how this change will ultimately benefit their patients.
Personalized Sales and Marketing Training
In addition to training your lab team to run the Calprotectin Chemiluminescence ELISA, ALPCO’s product management team will develop and provide a training package tailored for your organization’s sales and marketing needs. By training your sales and marketing teams about the advantages of using calprotectin testing to assist gastroenterologists with differentiating between IBD and IBS in their patients, you will successfully increase the value of your lab to the community. Some of the topics that can be covered in this training phase include:- Clinical utility of calprotectin relative to the differentiation between IBD and IBS
- Clinical accuracy of the Calprotectin Chemiluminescence ELISA versus other methods
- Use of calprotectin testing to reduce unnecessary colonoscopies for IBS patients

Go Live
Once your lab is ready to go live with the ALPCO Calprotectin Chemiluminescence ELISA, your entire organization will be prepared when samples start arriving. ALPCO is committed to providing you with the most clinically accurate calprotectin test so your lab can effectively assist gastroenterologists with diagnosing their patients with IBD and IBS. ALPCO’s Product Management, Customer Service, and Materials Management teams will work with your organization to arrange inventory availability and timely delivery of kits and extraction devices. ALPCO’s quality management system (QMS) is certified to the ISO 13485:2016 standard. Kits are designed and manufactured to yield accurate, reproducible results from plate-to-plate and batch-to-batch.
Post-validation Support
After your lab successfully implements the ALPCO Calprotectin Chemiluminescence ELISA, our organization will continue to provide refresher training to your existing team, training for new hires, and expert technical support.
Easily Validate the Calprotectin Chemiluminescence ELISA with ALPCO's Help
Although validation can be an overwhelming step in the assay implementation process, clinical labs need to continually transition to better technologies in order to remain competitive and provide impactful testing to the community. ALPCO’s Calprotectin Validation Assistance Program can help lower the barriers for clinical labs of all sizes seeking to run the most accurate FDA cleared fecal calprotectin test available. Our program is designed to provide your lab with assistance during every phase of validation to ensure your lab can successfully run the ALPCO Calprotectin Chemiluminescence ELISA. As part of our program, your organization will receive fully customized training, testing materials, on-site or remote assistance from our FAS during testing and data analysis, as well as post-validation support. Download a PDF of our Calprotectin Validation Assistance Program overview to share with your lab team members.
Download Program Overview
Download a PDF of our Calprotectin Validation Assistance Program overview to share with your lab team members.
Download Program Overview

