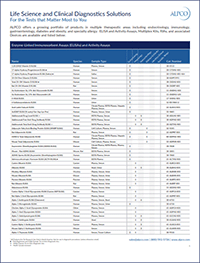
ELISAs for CHO Host Cell Proteins
Chinese Hamster Ovary (CHO) cells are the most dependable and most popular host cells for the industrial production of biotherapeutics. They are stable cell lines that are adaptable to a wide range of culture conditions and routinely produce very high protein and antibody yields. More importantly, CHO cells can produce complex proteins with post-translational modifications similar to human proteins. In addition to the protein or antibody of interest, a wide range of host cell proteins (HCPs) are secreted during CHO cell culture. More than 6,000 CHO host cell proteins have been identified. Some host cell proteins are produced at high concentrations and can co-purify with biopharmaceutical drug products. A subset of these proteins are considered particularly high risk due to immunogenicity, biological activity, or degradation of biotherapeutic product. Removal of host cell proteins from mammalian cell culture supernatants to trace levels is a critical step in the manufacture of biopharmaceuticals for human therapy.1,2,3,4,5 The measurement of relevant HCPs throughout the purification process can assist with process development and improve the safety, efficacy and stability of biotherapeutic products. ALPCO offers a growing line of quantitative immunoassays for the determination of specific CHO host cell proteins in culture supernatant samples.
Annexin A5 (NX5) Annexin A5 is a protein involved in apoptosis that binds with high affinity to phospholipids. It can elicit a strong immunogenic response when present in the final formulation of a therapeutic drug, making it a high risk HCP.3
Glutathione S-Transferase Pi (GSTp) Glutathione S-Transferase Pi is a cytosolic phase II enzyme involved in stress response and detoxification that has been flagged for potential immunogenicity in predictive models. GSTp is a persistent contaminant and one of the 30 most abundant HCPs identified in CHO antibody production.6,7,8
Legumain (LGM) Legumain was ranked 6th out of the 30 most abundant HCP in one study of batch and fed-batch CHO cultures. Legumain is a lysosomal protease that forms acidic charge variants by removing a functional group from asparagine residues (asparagine deamidation) of monoclonal antibodies, negatively impacting therapeutic antibody quality.6
Matrix Metalloproteinase 19 (MMP-19) MMP-19 is a proteolytic enzyme. It is considered a high risk CHO host cell protein due to its ability to cause antibody fragmentation and compromise the structural stability of therapeutic monoclonal antibodies.9
Nidogen-1 (NDN) Nidogen-1 glycoprotein is a CHO host cell protein found in abundance in both batch and fed-batch cultures. It is difficult to remove and negatively affects product quality.6,10
Phospholipase B-Like 2 PLBL2 is a frequently overlooked impurity that has been shown to coelute with several biotherapeutic antibodies during the purification process. Studies have demonstrated immunogenicity in humans.11
Looking for additional assays for CHO HCPs or automation options? Contact ALPCO to learn more.
References:
-
Harcum, Sarah, and Kelvin Lee. (2016). CHO Cells Can Make More Protein. PMID: 27883886
-
Kunert, Renate and David Reinhardt. (2016). Advances in recombinant antibody manufacturing. PMID: 26936774
-
Jones, M. et al. (2021). High-risk host cel proteins (HCPs): a multi-company collaborative view. Biotechnology and Bioengineering. 118(8):2870-2885.
-
Lavoie, R.A., et al (2019). Targeted capture of Chinese Hamster Ovary host cell proteins: peptide ligand discovery. Int. J. Mol. Sci. 20:1729; doi:10.3390/ijms20071729.
-
Molden, R., et al. (2021). Host cell protein profiling of commercial therapeutic protein drugs as a benchmark for monoclonal antibody-based therapeutic protein development. 13(1):e1955811.
-
Park, J.H. et al. (2017). Proteomic Analysis of Host Cell Protein Dynamics in the Culture Supernatants of Antibody-Producing CHO Cells. Nature: Scientific Reports. 7:44246. DOI: 10.1038/srep44246.
-
Valente, K.N., et al. (2018).Applications of proteomic methods for CHO host cell protein characterization in biopharmaceutical manufacturing. Current Opinion in Biotechnology. 53:144-150.
-
Wilson, L.J., et al. (2022). Identification and classification of host cell proteins during biopharmaceutical process development. Biotechnology Progress. 38:e3224.
-
Aboulaich, N. et al. (2014). A novel approach to monitor clearance of host cell proteins associated with monoclonal antibodies. Biotechnol. Prog. Vol. 30, No. 5.
-
Kol, S. et al. (2020). Multiplex secretome engineering enhances recombinant protein production and purity. Nature Communications. 11:1908
-
Vanderlaan M, et al. (2015). Hamster Phospholipase B-Like 2 (PLBL2). Bioprocess International. 13(4).