March 12, 2018
Investigating Immunogenicity of IBD Biologics
Biologics have been commonly prescribed as therapies for inflammatory bowel disease (IBD) since the late 1990s. However, research demonstrates that not all individuals respond to the first biologic taken and many lose response over time1. As a result, there is a great need to explore the immunogenicity of IBD biologics. Studies indicate that measuring drug levels, free anti-drug antibodies, and total anti-drug antibodies of common IBD treatment biologics is a key component to unraveling immunogenic responses to biologics2,3.
ALPCO offers a comprehensive portfolio of colorimetric therapeutic drug monitoring assays to investigate the immunogenicity of IBD biologics. ALPCO’s TDM assays are simple to use and generate quality data for IBD researchers.
 ALPCO offers therapeutic drug monitoring assays for investigating the immunogenicity of IBD biologics. Determining free anti-drug antibody and total anti-drug antibody levels allows for the analysis of primary non-response and loss of response to IBD biologics1,2,3. Furthermore, measuring drug levels of biologics can help 1,2,3. When used together, biologics and therapeutic drug monitoring assays have the potential to uncover deeper insights that may guide the development of new methods to improve the lives of those living with inflammatory bowel disease.
ALPCO offers therapeutic drug monitoring assays for investigating the immunogenicity of IBD biologics. Determining free anti-drug antibody and total anti-drug antibody levels allows for the analysis of primary non-response and loss of response to IBD biologics1,2,3. Furthermore, measuring drug levels of biologics can help 1,2,3. When used together, biologics and therapeutic drug monitoring assays have the potential to uncover deeper insights that may guide the development of new methods to improve the lives of those living with inflammatory bowel disease.
 Shop TDM ELISAs for IBD
Download our brochure to learn more about ALPCO's therapeutic drug monitoring assays for investigating the immunogenicity of IBD biologics.
Download Brochure
Shop TDM ELISAs for IBD
Download our brochure to learn more about ALPCO's therapeutic drug monitoring assays for investigating the immunogenicity of IBD biologics.
Download Brochure
Investigating Immunogenicity of IBD Biologics
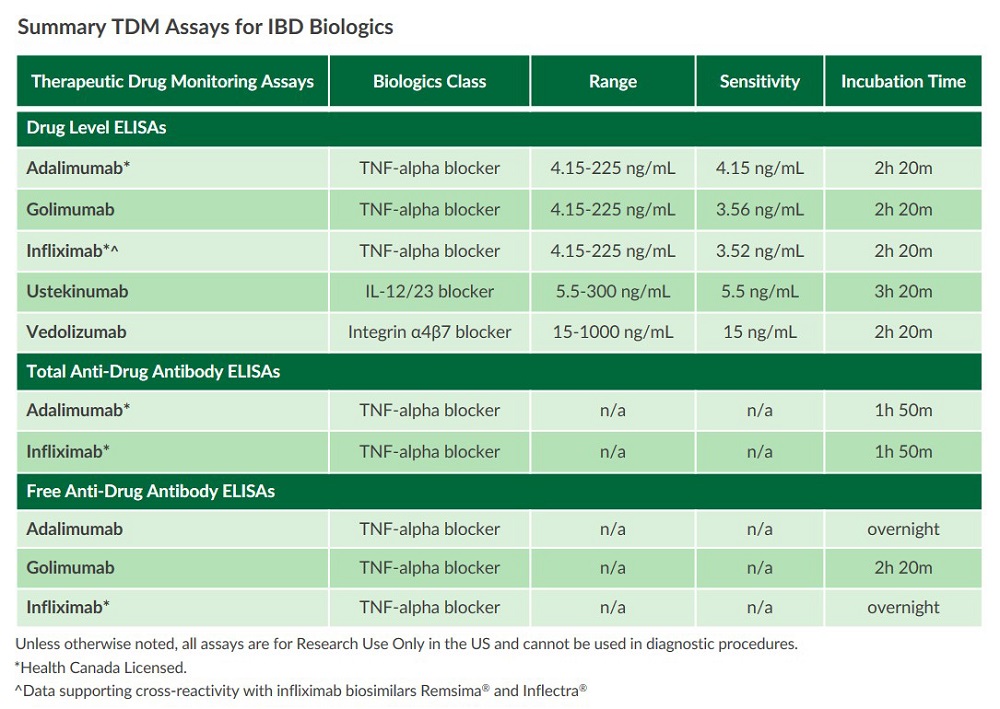
Therapeutic drug monitoring assays (TDM assays) can measure the drug, free anti-drug antibody (ADA), and total anti-drug antibody levels of common biologics classes used to treat IBD including TNF-alpha, integrin antagonists, and interleukin blockers. Drug level and ADA measurements can aid in the investigation of primary non-response (PNR) as well loss of response (LOR) to biologics therapies such as:- Adalimumab
- Infliximab
- CT-P13 (infliximab biosimilar)
- Golimumab
- Vedolizumab
- Ustekinumab
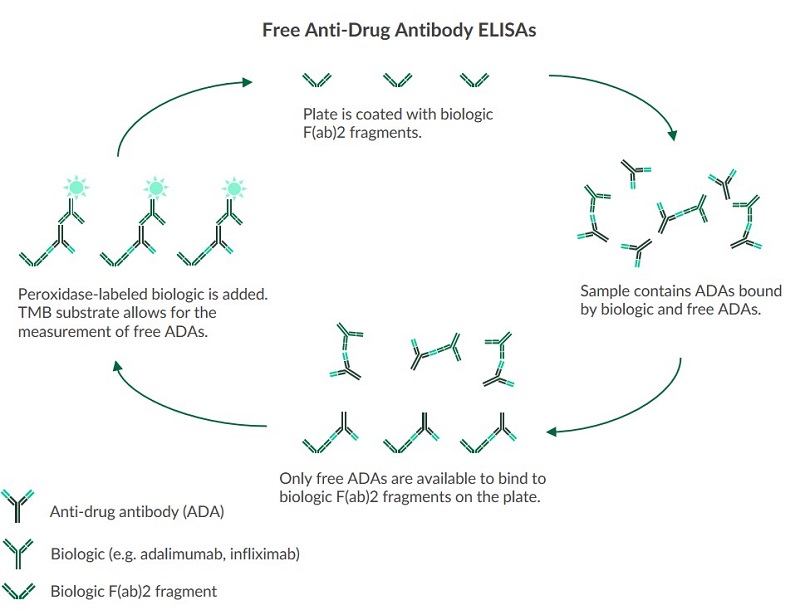
Free Anti-Drug Antibody ELISAs
The measurement of free anti-drug antibodies with TDM assays allows for the semi-quantitative determination of unbound antibodies against a given biologic which enables an assessment of the immune system’s response to a given biologic. Free anti-drug antibody assays can be used to semi-quantitatively determine the unbound anti-drug antibodies against three TNF-alpha blockers:- Adalimumab*
- Infliximab*
- Golimumab*

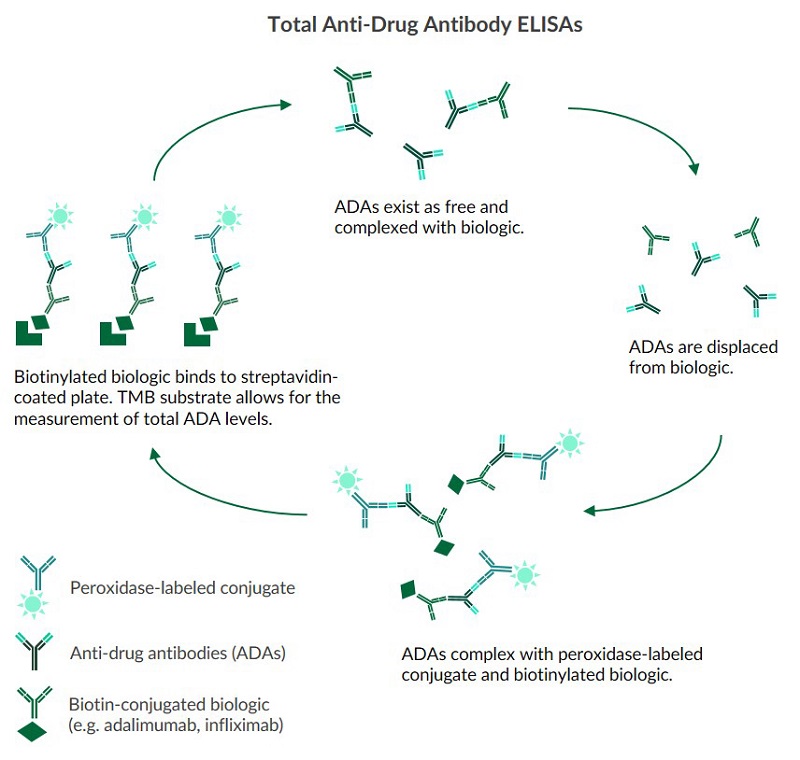
Total Anti-Drug Antibody ELISAs
The measurement of free and bound antibodies allows for the semi-quantitative determination of total ADA levels in circulation. and can help explain whether or not antibodies are playing a role in PNR and LOR to a given IBD biologic. Total anti-drug antibody assays can be used to semi-quantitatively determine the total anti-drug antibody levels of two common TNF-alpha blockers:- Adalimumab*
- Infliximab*

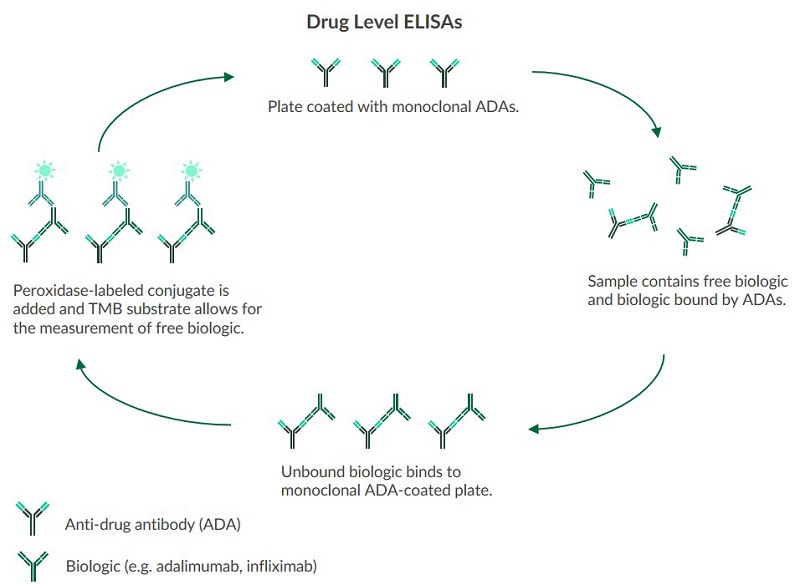
Drug Level ELISAs
Drug levels for biologics can be measured to help investigate the optimal concentrations of a given biologic. Understanding the optimal drug concentration can help avoid doses that are too low (ineffective) or too high (toxic). Drug level assays can quantify levels of:- Adalimumab*
- Infliximab*
- Golimumab
- CT-P13
- Vedolizumab
- Ustekinumab
 ALPCO offers therapeutic drug monitoring assays for investigating the immunogenicity of IBD biologics. Determining free anti-drug antibody and total anti-drug antibody levels allows for the analysis of primary non-response and loss of response to IBD biologics1,2,3. Furthermore, measuring drug levels of biologics can help 1,2,3. When used together, biologics and therapeutic drug monitoring assays have the potential to uncover deeper insights that may guide the development of new methods to improve the lives of those living with inflammatory bowel disease.
ALPCO offers therapeutic drug monitoring assays for investigating the immunogenicity of IBD biologics. Determining free anti-drug antibody and total anti-drug antibody levels allows for the analysis of primary non-response and loss of response to IBD biologics1,2,3. Furthermore, measuring drug levels of biologics can help 1,2,3. When used together, biologics and therapeutic drug monitoring assays have the potential to uncover deeper insights that may guide the development of new methods to improve the lives of those living with inflammatory bowel disease.
 Shop TDM ELISAs for IBD
Download our brochure to learn more about ALPCO's therapeutic drug monitoring assays for investigating the immunogenicity of IBD biologics.
Download Brochure
Shop TDM ELISAs for IBD
Download our brochure to learn more about ALPCO's therapeutic drug monitoring assays for investigating the immunogenicity of IBD biologics.
Download Brochure
References
- Roda et al. (2016). Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol. 2016 Jan;7(1):e135. doi:10.1038/ctg.2015.63. PMCID:PMC4737871.
- Krishna et al. (2016). Immunogenicity to Biotherapeutics – The Role of Anti-drug Immune Complexes. Front Immunol. 2016;7:21. PMCID: PMC4735944.
- Kopylov et al. (2014). Therapeutic drug monitoring in inflammatory bowel disease. Ann Gastroenterol. 2014;27(4):304-312. PMCID: PMC4188926.

