Enhance Your Lab’s Molecular GI Portfolio
Molecular GI testing for bacteria such as salmonella, vibrio, and E. coli, and viral pathogens including norovirus and rotavirus, is instrumental in the diagnosis and treatment of infection and dysbiosis. In addition, many gastrointestinal disorders often require protein biomarker tests to aid in accurate detection and diagnosis. ALPCO has worked with several clinical testing labs to incorporate common stool-based protein biomarkers into their menu, achieving average growth of 120% in testing volumes over four years.
The Advantages of Adding ALPCO Protein Biomarker Tests
Complementary tests: Stool-based protein biomarker tests can be marketed to existing customers for molecular GI panels as an aid in the diagnosis of GI disorders (especially non-pathogen-related). Accepted clinical tests: A number of in vitro diagnostic stool-based tests, including calprotectin and pancreatic elastase, are widely used and accepted by physicians and gastroenterologists. Flexible sample collection: Single stool samples can be collected in a clinical setting or at home, simplifying sample collection and patient participation. Common sample preparation procedure: Most ALPCO GI biomarker tests are performed from a single stool extract prepared from a raw stool sample. Our universal stool extraction device eliminates manual weighing for most stool samples, simplifying sample preparation.
Pancreatic elastase (PE): Our Pancreatic Elastase ELISA is a 510(k)-exempt assay for in vitro diagnostic use as an aid in the detection of exocrine pancreatic insufficiency (EPI). EPI can be caused by a range of disorders affecting the production or release of pancreatic enzymes required for proper digestion and nutrient absorption. Research studies have associated levels of PE between 100 and 200 µg/g with moderate EPI and levels below 100 µg/g with severe EPI.
Calprotectin: Our Calprotectin Chemiluminescence ELISA is FDA 510(k)-cleared and Health Canada licensed for in vitro diagnostic use as an aid in the diagnosis of inflammatory bowel disease (IBD) and as an aid in the differentiation of IBD from irritable bowel syndrome (IBS) in conjunction with other clinical and laboratory findings. ALPCO offers the most clinically accurate 510(k)-cleared calprotectin test on the market today. When compared to other calprotectin tests, our assay reduces false positives for IBD from 20 – 33% to just 7.5%.
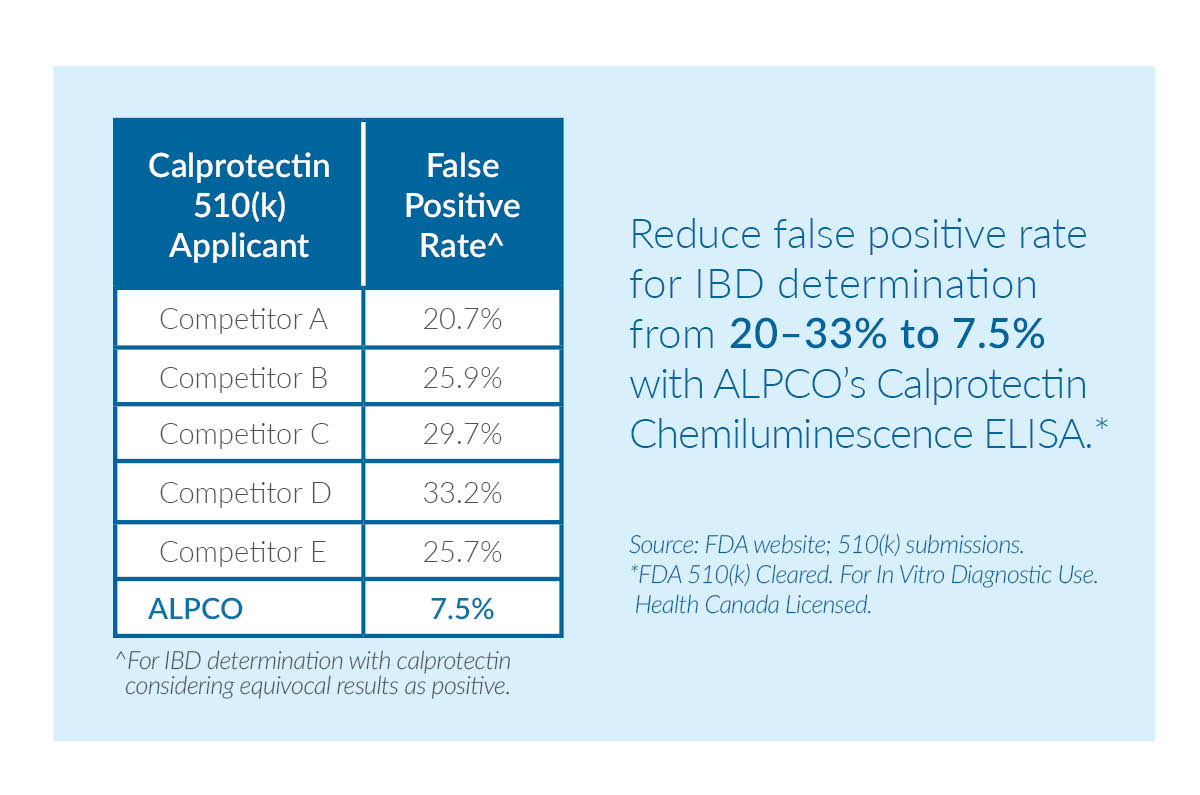
Figure 1: When compared to five different calprotectin tests, ALPCO's assay has the lowest false positive rate for IBD determination.
Implementation and Validation Assistance
ALPCO makes your switch simple. Our convenient Validation Assistance Program enables your lab to get up and running quickly with stool-based protein biomarker tests. We can assist with validation kits, stool extraction devices, well-characterized sample panels, workflow optimization, and in-lab support.
Request a Meeting or a Free Evaluation Kit*
Submit the form below to learn more about implementing stool-based biomarker tests.
*Evaluation kits are available to customers in North America only. Limit one evaluation kit per laboratory.
