September 27, 2016
Regulating Endocrine Disrupting Chemicals in Consumer Products
Date: September 27, 2016
Author: Andrea Tarbet, Product Marketing Associate at ALPCO
From cosmetics to water bottles, endocrine disrupting chemicals (EDCs) have surfaced in our everyday lives. EDCs are compounds that can interfere with the endocrine system’s ability to help an organism develop, grow, reproduce, and maintain overall homeostasis1. Since EDCs are found nearly everywhere, we are constantly exposed to mixtures of multiple EDCs2,3.
More and more frequently, product ingredient warnings are featured on newscasts and in magazine articles. This summer, sunscreen ingredients came under fire due to research findings indicating that common chemicals used as UV filters, such as oxybenzone, can mimic estrogen and disrupt the endocrine system4. Since these ingredient warnings are becoming so frequent, some are wondering how these products make it to the market in the first place. Why aren’t these issues addressed before the products are available to consumers?
Challenges with Regulating Endocrine Disrupting Chemicals
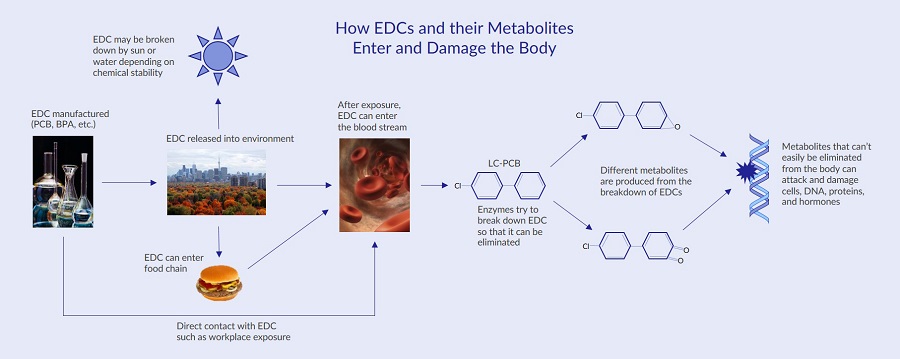
Products containing chemicals are regulated by different U.S. government agencies. Both the U.S. Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) enforce laws to ensure chemicals and products are safe for consumer use. Since their establishment, both the EPA and FDA have made huge strides in the laws protecting consumers against harmful substances, such as the 1976 Toxic Substances Control Act (TSCA) and the 1938 Federal Food, Drug, and Cosmetic (FDC) Act. Despite the EPA and FDA working to provide consumers with safe products, there are several challenges specifically associated with regulating endocrine disrupting chemicals in consumer products1. These challenges were discovered due to the investigation of different EDCs in various human and animal studies:Metabolites of Endocrine Disrupting Chemicals
Endocrine disrupting chemicals can be degraded by the environment or by enzymes in the body after an EDC is ingested. Research suggests that the metabolites generated during the break down of EDCs may also be harmful11. For instance, a large number of different polychlorinated biphenyls (PCBs) used to be manufactured in the U.S. Since so many types of polychlorinated biphenyls were manufactured, many different metabolites form when the compounds are broken down. One group of PCBs called lower chlorinated PCBs (LC-PCBs) are broken down quickly in the blood11 leading to various metabolites and free radicals that can attack and damage DNA, RNA, and proteins12.
Delayed Effects of Endocrine Disrupting Chemical Exposure
Sometimes the effects of endocrine disrupting chemicals in consumer products aren’t discovered until long after they’ve been on the market. A prime example of this is diethylstilbestrol (DES), a type of synthetic estrogen, prescribed between 1938 and 1971 to prevent pregnancy complications. It wasn’t until 15-20 years after mothers took DES that the harmful side effects surfaced when their daughters began developing a rare cancer called clear cell adenocarcinoma (CCA) early in their lives5.Developmental Stage of Endocrine Disrupting Chemical Exposure
Human development involves several stages sensitive to endocrine disrupting chemicals, including organogenesis and puberty6. Research from human and animal studies has demonstrated how exposure to bisphenol A (BPA) at different stages can hinder growth and increase health risks. These effects include prenatal malformation of male and female genitalia, childhood brain development impairments, and links to obesity in adults7.Epigenetic Changes
Scientists have discovered evidence suggesting that EDCs can modify gene expression without mutating DNA, also known as epigenetic changes8. Gene expression can be modified by DNA methylation, histone modifications, and fluctuation in the levels of non-coding regulatory RNAs. Epigenetic changes can impact germlines, therefore increasing the risk of passing these issues on to subsequent generations. For example, research has shown that diethylstilbestrol not only affected daughters of women who took the drug, but also their sons and grandchildren9,10.Overcoming Challenges in Regulating Endocrine Disrupting Chemicals
These are just a few of the challenges associated with regulating endocrine disrupting chemicals in consumer products. When considering these issues, it is clear that this is a growing concern. One way to help overcome these challenges is to continue to research and establish a standard of biomarkers to investigate the effects of EDCs in both animal and human models. Additionally, the passing of new acts by both the FDA and EPA requiring more stringent testing of new chemicals can help to keep our endocrine systems in balance by preventing EDCs like diethylstilbestrol and polychlorinated biphenyls from entering the market. References- Diamanti-Kandarakis et al. (2009). Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev., 30(4), 293-342. PMID: 19502515.
- Crews et al. (2003). Wildlife as models for the study of how mixtures, low doses, and the embryonic environment modulate the action of endocrine-disrupting chemicals. Pure Appl. Chem., 75(11–12), 2305–2320. DOI: 10.1351/pac200375112305.
- Molehin et al. (2016). Prenatal exposures to multiple thyroid hormone disruptors: Effects on glucose and lipid metabolism. J. Thyroid Res., 2016. PMID: 26989557.
- Maipas et al. (2015). Sun lotion chemicals as endocrine disruptors. Hormones (Athens), 14(1), 32-46. PMID: 25885102.
- Herbst et al. (1971). Adenocarcinoma of the vagina: Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med, 284, 878-881. PMID: 5549830.
- Gore et al. (2015). EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocrine Reviews, 36, E1–E150. PMID: 26544531.
- Mikołajewska et al. (2015). Bisphenol A: Application, sources of exposure and potential risks in infants, children and pregnant women. Int. J. Occup. Med. Environ. Health, 28(2), 209–241. PMID: 26182919.
- Cruz et al. (2014). Long-term effects of early life exposure to environmental estrogens on ovarian function: Role of epigenetics. J. Neuroendocrinol., 26(9), 613-24. PMID: 25040227.
- Reed & Fenton. (2013). Exposure to diethylstilbestrol during sensitive life stages: A legacy of heritable health effects. Birth Defects Res. C Embryo Today, 99(2). PMCID: PMC3817964.
- Hilakivi-Clarke. (2014). Maternal exposure to diethylstilbestrol during pregnancy and increased breast cancer risk in daughters. Breast Cancer Res., 16(2), 208. PMID: 25032259.
- Grimm et al. (2015). Metabolism and metabolites of polychlorinated biphenyls (PCBs). Crit. Rev. Toxicol., 45(3), 245–272. PMCID: PMC4383295.
- Agency for Toxic Substances and Disease Registry. (2016). Polychlorinated Biphenyls (PCBs) Toxicity: What Are Adverse Health Effects of PCB Exposure? Agency for Toxic Substances and Disease Registry.
